The medical programme is the result of proper development and stems from many years of experience in pressure sensor production. The most extensive is the invasive blood pressure measurement programme, trademarked hybymed® and standalone components which are also available for further installation.
An ISO 13485 certified and maintained quality management system and proper production in clean room (Class 8 by ISO 14644-1) guarantee the high and consistent quality of our products.
A detailed assortment of hybymed® products is available in the catalogue, or you may contact our Customer Support Service.
All hybymed® products are CE certified.
REGULATORY COMPLIANCE
Hybymed® SYSTEM and IBPM PLUS SYSTEM are compliant with the European 93/42/EEC Directive (Medical Devices Directive – MDD), IEC 60601-1 and IEC 60601-2-34.
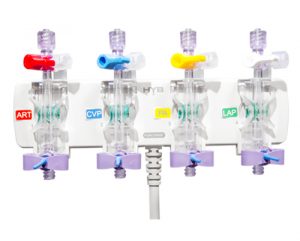
INVASIVE BLOOD PRESSURE MEASUREMENT
Hybymed® invasive blood pressure measurement (IBPM) medical devices offer an optimal solution for blood pressure measurement in human medicine. The products are used in hospitals during surgery, in invasive therapy departments, and cardiology departments. Hybymed® medical devices are of a high quality, reliable, simple to use, and CE certified.
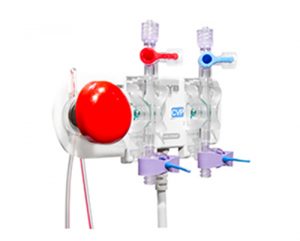
Besides basic blood pressure measurement by several IBPM system variants, the products additionally enable a closed blood sampling system IBPM PLUS system. Components and accessories simplify everyday use.
The products are also available in versions prepared according to specific customer’s wishes.
HYBYMED® PRODUCTS
IBPM SYSTEM
IBPM PLUS SYSTEM
COMPONENTS AND ACCESSORIES
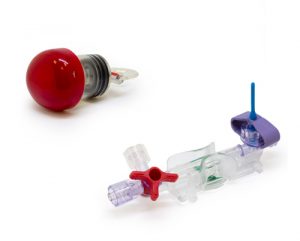
STANDALONE COMPONENTS
The standalone components are intended for further installation in various systems and are not available for direct use in hospitals. The most demanded are the pressure sensor and the reservoir for closed blood sampling system.